What are they?
Let’s take a look at the slow release nutrient granules. They have gained increased popularity in the hobby as a cheap and easy alternative for root tabs. These granules, from various manufacturers, are designed to provide macro- and micronutrients for potted plants for 6 months. That sounds perfect! Mostly you will see them as (re)sold in gel caps that can easily be pushed in the substrate instead of adding individual beads. Adding some when setting up the tank should provide a slow, constant but rich source of nutrients to the plant roots. Exactly where the plants need them. After 6 months, add some more granules in an easily decomposing capsule and we are done. No need for expensive aquarium soils, root tabs or skipping over the “plants that need a rich substrate” even in an aquarium with sand. We even heard accounts where adding very high amounts of these nutrient grains to the substrate allowed the growth of plants that would have stunted in a rich water column (water column tests were not done or not published) .
Many of us have kept or have read about aquariums with soil and a sand cap. We know that ammonia leaks from the soil into the water column in the first stages. But we expect that the nutrients from these granules will stay out of the water column. Is this really the case? If they slowly release their nutrients perhaps the levels will be so low that we cannot even detect them. But do they last 6 months in our aquariums? A first look at the manufacturer’s description says the rate of release depends on temperature and humidity.
There are many reports where the amount of slow-release nutrient capsules was overdosed and the aquarium had a large algae outbreak. At the same time, seems that exposing a large amount of these granules to the water, by uprooting, for example, leads to the same algae growth.
Aims
Some claimed that they are able to grow great plants just by adding these time-released capsules and no water column fertilizers. But if these granules release nutrients into the water column, is there really a case of no water column fertilizers?
Does an undisturbed sand-cap allow any nutrients to escape in the water column?
What is more, we don’t yet know exactly what is released and for how long do they keep releasing it. Clearly they are not designed to be continuously under-water , does this affect their 6 months life-time?
Thus we want to find out if they release nutrients in the water column, what they release and how fast they deplete.
Experimental design
When we setup a tank we clearly do not plan to wait a few days / weeks to see if anything from the nutrient granules is released in the water column. Bacteria, plant and algae uptake make it hard to detect what is actually released from these granules. To make things even harder on us, we use tap water or remineralized reverse osmosis water which introduces its own substances. We add different substrates, fertilizers or fish food etc. We cannot say what comes from where.
To avoid this we have 3 aquariums A, B, C. Aquariums A and C will have 13.3g of time-released nutrient granules. Aquariums A and B will have a 5cm deep sand bed. In this way we can compare how a sand cap influenced the released of nutrients in the water column (compare between A and C). We can also see if sand alone added something to the water (B). And we can see how fast the capsules release their nutrients and what nutrients they release (B vs C).
The nutrient granules advertise 6 months worth from one application and have a stated chemical analysis of : 17% total nitrogen (7.7% NO3-N; 9.3% NH3-N); 15.5% P2O5; 11% K2O; 2% MgO; 0.01% B; 0.025% Cu; 0.22% Fe; 0.03% Mn;0.01% Mo; 0.008% Zn.
All 3 aquariums were filled with 10L RODI (reverse osmosis deionization) water with a EC (electrical conductivity) of 20 µS cm-1 . All aquariums are aerated with new airstones from the same pump. No light is provided for the duration of the experiment. No plants or animals are added to the aquariums.
After 21 days, water parameters were measured. The water in the aquariums with nutrient grains was changed with RODI water , with a conductivity of 0 µS cm-1 . The original granules and subtrate were left in place.
Results
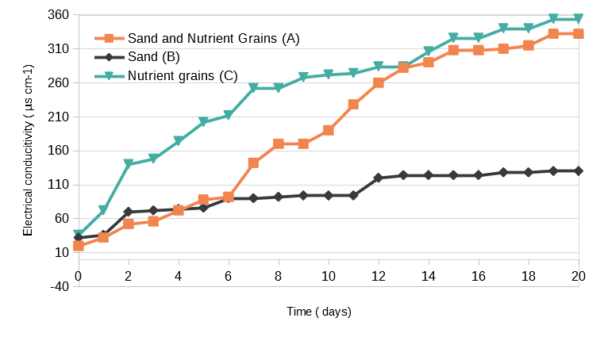
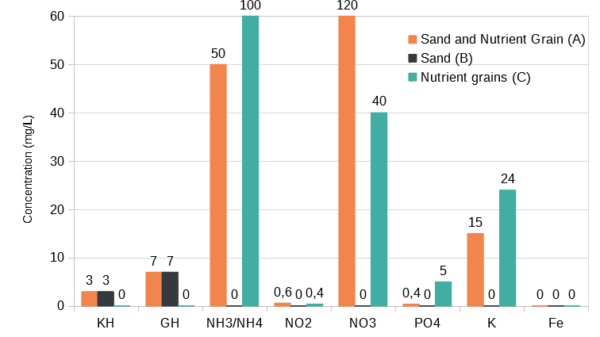
The nutrient grains release large amounts of ammonia and phosphate in the water column. With them fully exposed to the water column the electrical conductivity increase in 10 days was of 232 µS cm-1 . A high increase of ammonia and phosphate levels was detected. Iron levels were non-detectable .
The addition of the sand cap slowed down this process to some degree, but still allowed high concentrations of ammonia (above 5mg/L) to go into the water column . Within the first 3 days, ammonia was already present in the water column of the sand covered nutrient grains.
At 7 days, nitrate was also detected. The source of nitrate is both from the nutrient grains and by nitrification from the ammonia released. The presence of nitrite, the lack of any detectable nitrate at first and the increased levels of nitrate in the tank with a sand substrate points towards a high contribution of nitrification.
Potassium was also present in both aquariums with nutrient grains but not in the aquarium with sand only.
Very low levels of phosphate were detected in the aquarium with nutrient grains covered by sand. As the sand is clearly a source of soluble calcium it is possible that some phosphate precipitated before leaving the sand.
No iron was detected in either of the aquariums.
Sand is mostly considered an inert substrate. This experiment shows that not all sand is created equal. While not contributing any nitrate or phosphate, the sand-only experiment released enough substances in the water column to raise the electrical conductivity by 130µS cm-1 . This is supported by the increase in GH and KH as Ca2+, Mg2+ and , –HCO3.
Discussion
It seems that sand capped nutrient granules still contribute a great amount of ammonia to the water column, resulting in values far from those of lean water column. Plants readily take up ammonia, easier than nitrate. Unfortunately, a high concentration of ammonia is also a trigger for algae. This explains the struggles some report with using nutrient granules. Such a big ammonia release within the first couple of weeks when the biological filter is just getting established means ammonia is always available in the water column. This has the potential of causing more problems than a simple sand only aquarium.
No iron was detected in either of the aquariums. This may become available in anaerobic conditions or by direct root contact, but could also be at very low concentration in the granules or precipitated already.
While they release relatively high concentrations of macronutrients in the water column, they do so over several weeks. This slower realease may still find applications in the aquariums , especially in aquariums with no fish and low pH.
Possible uses of the nutrient granules include :
- providing a source of ammonia for tank “cycling “
- source of longer term fertilization during extended holidays
In these applications care must be taken to not add too many grains. Looking at the data from this experiment 2g in 100L can be enough to sustain a population of ammonia oxidizing bacteria when starting a tank or maintaining a tank without fish for a period. Preferably, these grains should be burried in a separate container that can easily be removed or in a filterbag in the filter or the aquarium. Quick removal of the grains is important before the addition of fish.
Annex
Table 1. Measured parameters in the 3 aquariums.
| Day | EC | Temp | NO3 | NH3 | PO4 | K | Fe | GH | KH |
| 0 /15. Dec | A -20 B- 32 C- 36 | 22 °C 22 °C 22 °C | 0 0 0 | 0 0 0 | 0 0 0 | 0 0 0 | 0 0 0 | – | – |
| 1 | A -32 B- 36 C- 72 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 2 | A -52 B- 70 C- 140 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 3 | A -56 B- 72 C- 148 | 22 °C 22 °C 22 °C | 0 0 1 | 0,5 0 10 | 0 0 >1.8 | – | 0 0 0 | 2 3 0 | 1.5 1.2 0 |
| 4 | A-72 B-74 C-174 | – | – | – | – | – | – | – | – |
| 5 | A-88 B-76 C-202 | 20°C 20°C 20°C | – | – | – | – | – | – | – |
| 6 | A-92 B-90 C-212 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 7 | A-142 B-90 C-252 | 22 °C 22 °C 22 °C | 10 0 10 | 5 0 >10 | – | – | – | – | – |
| 8 | A-170 B-92 C-252 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 9 | A-170 B-94 C-268 | 22 °C 22 °C 22 °C | 20 0 10 | 5 0 est.50 | 0 0 6 | – | 0 0 0 | 4 4 0 | 3 3 0 |
| 10 | A-190 B-94 C-272 | – | – | – | – | – | – | – | – |
| 11 | A-228 B-94 C-274 | – | – | – | – | – | – | – | – |
| 12 | A-260 B-120 C-284 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 13 | A-282 B-124 C-284 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 14 | A-290 B-124 C-306 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 15 | A-308 B-124 C-326 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 16 | A-308 B-124 C-326 | – | – | – | – | – | – | – | – |
| 17 | A-310 B-128 C-340 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 18 | A-315 B-128 C-340 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 19 | A-332 B-130 C-354 | 22 °C 22 °C 22 °C | – | – | – | – | – | – | – |
| 20 | A-332 B-130 C-354 | 22 °C 22 °C 22 °C | 40 0 120 | 50 0 100 | 0.4 0 5 | 15 0 24 | 0 0 0 | 3 3 0 | 7 7 0 |